Hopantenic acid based products are widely used in neurological practice. But not all patients know what Pantogam and its analogues are prescribed for, and what effect these drugs can have on their bodies. We suggest making your own conclusions based on the information presented in the official instructions.
Material Content:
Description of release forms and composition
The drug in question has two dosage forms - this is syrup and tablets. The liquid form is used mainly in children under three years of age, the rest are prescribed tablets.
Some doctors even prescribe a tablet form to a younger group of patients. This is because syrup often provokes the development of negative reactions.
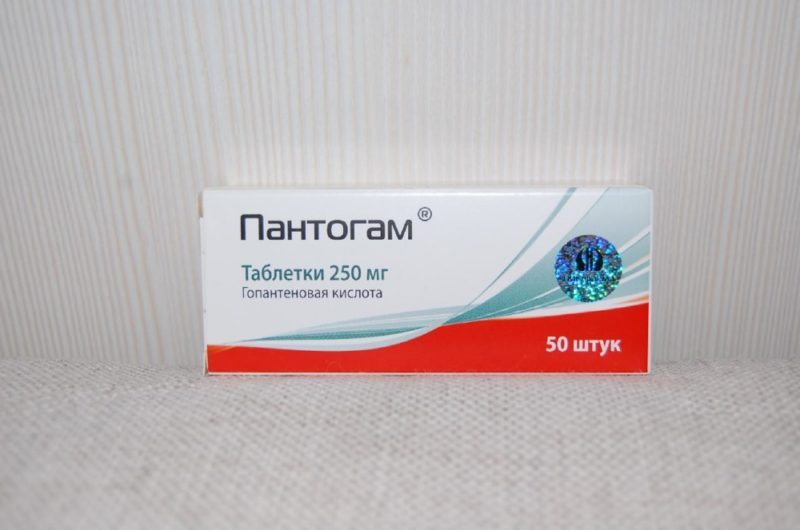
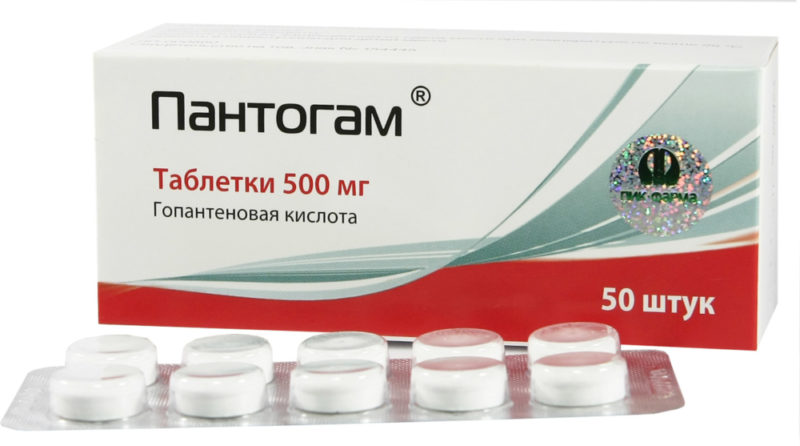
Both forms contain homopantothenic acid salt as the active substance. In tablets, its amount is 250 or 500 mg. In syrup - 10%.
In the pills, additional components are used that are responsible for maintaining the dosage form. The total mass of such components is 60 mg.
The pills are white inside and out. They have a flat cylindrical shape. It tastes very bitter. Easily split in half thanks to the risk.
Packed in blisters made of PVC film and foil. The outer packaging is a cardboard box. The tablets are stored for no more than 4 years.
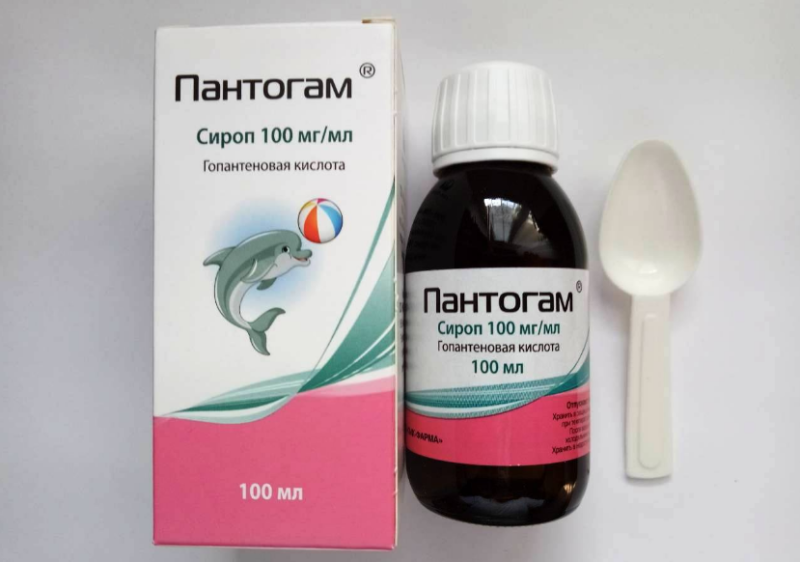
In syrup, auxiliary components are: glycerol, citric acid, sodium benzoate, sweeteners sorbitol and aspartame, as well as an artificial cherry flavor. In a 100 gram vial, the mass of all components, including the active substance, is 51.06 g. The remainder is purified water.
The syrup has a slightly sweet taste, a yellowish color and a characteristic cherry smell. It is bottled in tinted glass bottles with a capacity of 100 ml. There is a tamper ring on the bottle under the cap.
An additional packaging for the medicine is a dense box. In addition to the annotation, a plastic spoon with divisions of 1.25 and 2.5 ml or 2.5 and 5 ml is inserted into each pack. The shelf life of the syrup is two years.
What is Pantogam prescribed for?
The tool is used only on the territory of those countries where the procedure for placebo-controlled trials is not mandatory. To date, no such studies have been conducted with respect to this drug. All data on its effectiveness are based on observations obtained during treatment of patients.
In Europe and the USA, this drug is not approved for use. However, our doctors have prescribed it for more than 20 years.
According to the manufacturer, the drug exhibits nootropic properties, that is, it protects nerve cells from negative effects, helps restore nerve connections, and also improves the transmission of electrical impulses.
It is a metabolism, that is, it regulates metabolic processes. But it does not show anabolic properties - it is not able to rebuild damaged tissues. Protects the brain from the effects of oxygen starvation, organic damage due to bruises, age-related changes and the effects of toxic compounds.
The effectiveness of the active component is explained by the presence in its chain of a residue of gamma-aminobutyric acid (GABA), which is the main mediator of inhibition reactions in the central nervous system (CNS). The tool simultaneously has a sedative and tonic effect. Relieves excessive excitability, convulsions, suppresses obsessive motor activity. At the same time, it strengthens concentration, mental and physical performance.
Restoring the level of GABA, the drug alleviates the condition during alcohol withdrawal in patients with chronic intoxication.
It is prescribed for adults and children in certain conditions:
- impaired mental ability, as a result of traumatic brain injury or neuroinfection;
- mental disorders (mental retardation, schizophrenia);
- disruption of the brain as a result of damage to its vessels;
- motor dysfunctions caused by neural disorders (epilepsy, convulsions, parkinsonism, tremor, stuttering);
- lack of attention and analytical skills as a result of emotional overload;
- impaired urination of a neurogenic nature (incontinence, enuresis);
- consequences of birth injuries, hypoxia and infectious lesions during pregnancy (impaired speech, psychomotor development or a combination thereof; cerebral palsy, tics, stuttering, neurosis, hyperactivity).
There is evidence of the ability of calcium hopantenate to exhibit analgesic properties in the defeat of the trigeminal nerve.
Instructions for use, dosage of syrup and tablets
Instructions for use allow you to prescribe a nootropic agent from the first days of life.
Doctors note that the main percentage of side effects occur precisely in infants. In children, after a year of such reactions practically does not occur.
For adults, the drug is prescribed much less often, since for this category of individuals a wider line of effective nootropics has been developed.
For children
Children can be prescribed both Pantogam syrup 100 mg / 1 ml, and tablets in a dosage of 250 and 500 mg. The medicine should be taken half an hour after a meal.The duration of treatment is determined individually and ranges from one to six months.
Start treatment with the lowest possible dose, bringing it to therapeutic values within 7 to 12 days. A week before the end of the course, the dose is again reduced, leading to a gradual withdrawal of the drug.
Depending on the age of the patient, for the medicine in the form of syrup, the following daily dose ranges exist:
- child up to a year is shown from 5 to 10 ml;
- up to three years, the maximum volume of the drug is 12.5 ml;
- by seven years, the maximum amount of funds should not exceed 15 ml;
- up to 14 years of age no more than 30 ml of syrup is indicated.
A single dose varies from 2.5 to 5 ml. In order to avoid problems with falling asleep, the drug is recommended to be taken in the morning and afternoon.
In case of urination disorders, children are prescribed up to three age-related doses of syrup per day.
Tablets, similarly in liquid form, are taken after a meal. For children, the daily amount is from 0.75 to 3 g. At one time, from 0.25 to 0.5 g.
The frequency of administration depends on the nature of the pathology:
- involuntary obsessive movements that appeared during treatment with psychotropics - from 3 to 4 times;
- tics - 3 to 6 doses per day;
- urination disorders - up to three times a day;
- pathology of the nervous system - up to six doses per day.
Some diseases, such as epilepsy, can be treated for more than a year. Before re-conducting the course, take a break of six months.
For adults
Adults can take medicine in the form of syrup and tablets. The amount of liquid medication should be no more than 10 ml at a time and no more than 30 ml total per day.
- In the treatment of mental disorders, in combination with psychotropics, up to 30 ml of syrup is prescribed per day.
- With epilepsy, together with anticonvulsants, take up to 10 ml of the drug.
- For various motor disorders, up to 30 ml of syrup is indicated.
- During the recovery period after traumatic brain injury take no more than 30 ml of the drug.
- To combat the effects of mental overload, up to 15 ml of syrup is prescribed.
- Urination disorders are treated with a dose of up to 30 ml per day.
The multiplicity of reception is determined by the indications for use:
- epilepsy - up to 2 pcs. 500 mg each;
- motor disorders - up to six tablets of 500 mg per day;
- with traumatic brain injuries - Pantogam tablets 250 mg, up to 4 times;
- to remove the effects of antipsychotic treatment - 1 to 2 tablets of the highest dosage up to three times a day;
- during treatment of urination disorders, from 3 to 6 tablets with the highest concentration of active substance are distributed in two to three doses.
In view of the possible stimulating effect, the main amount of the drug should be in the afternoon hours.
During pregnancy and lactation
For the drug in the form of tablets, pregnancy and lactation are direct contraindications. Syrup is forbidden to take only in the first trimester of pregnancy. Lactation for this dosage form is not included in the list of contraindications.
Drug interaction
Medicines, the effect of which "Pantogam" enhances:
- barbiturates;
- anticonvulsants;
- glycine;
- ethidronic acid;
- procaine.
It is noted that Pantogam is able to prevent the occurrence of side effects from treatment with antipsychotics.
Contraindications, side effects and overdose
Among the contraindications: sensitivity to the components of the syrup and tablets, as well as serious kidney pathologies in the acute phase.
The bulk of side effects occur in infancy. Among the negative reactions there is an allergy. In most cases, it is caused by syrup. Sensitivity can manifest as an allergic rhinitis, inflammation of the conjunctiva of the eye, rashes, and skin itching.
More often, children under one year old, less often other categories of people experience neurological disorders, such as sleep disturbance, increased irritability, anxiety, headache, or, conversely, a depressed state, lethargy, and drowsiness.
When adverse reactions occur, a transition from one form of the drug to another is possible. If this does not help, it is necessary to reduce the dose or completely cancel the medicine.
With an overdose of the drug, side effects increase. This condition requires gastric lavage, intake of sorbents and the appointment of therapy to relieve symptoms.
Analogues of the drug
The following drugs are available on the basis of hopantenic acid:
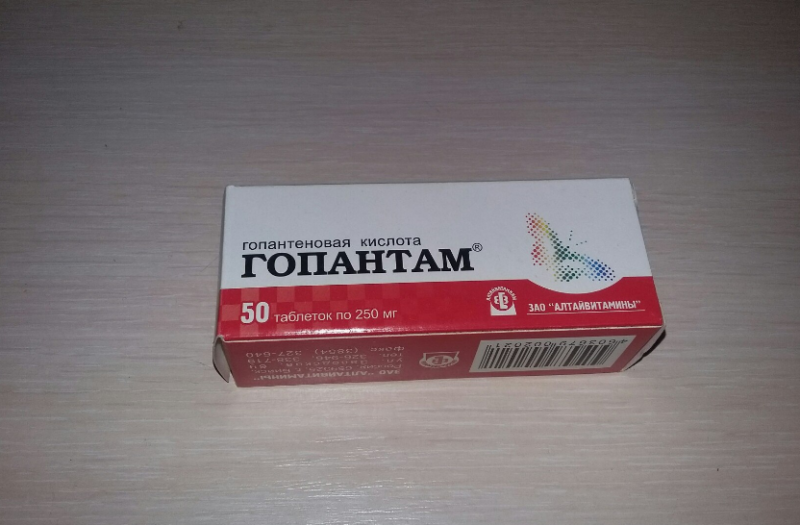
- "Gopantam", nootropic in the form of tablets of 0.25 and 0.5 g;
- Calcium hopantenate tablets with anticonvulsant effect (250 mg);
- "Pantocalcin" is a tablet form of hopantenic acid in 0.25 and 0.5 mg.
“Pantogam Asset” 300 mg and 200 mg in the form of capsules, including a modified molecule of hopantenic acid, can be attributed to analogues.
Parents of children who were prescribed Pantogam notice that this drug is prescribed for a very wide range of conditions. It is recommended both to patients with overt abnormalities and to completely healthy children.
What this policy is based on is unknown. Perhaps this is a consequence of the effectiveness of the drug or just the result of marketing calculations by the manufacturer. In any case, the lack of a placebo-controlled trial casts doubt on the safety of this drug.